The Museum Blog
Category: Junior Science
Spooky Science: Disappearing Ghosts
Disappearing Ghosts
Materials Needed:
- Biodegradable packing peanuts (made of corn!)
- Dark colored marker
- Pipette or paintbrush
- Small cup of water
- Tray/plate/cookie sheet
Instructions - Set-up:
- Use your marker to draw ghosty faces on several biodegradable packing peanuts
- Invite your young scientist(s) to draw some faces, too!
- Place ghosts, small cup of water, and pipette on a tray, plate, or cookie sheet
Instructions - Activity:
- Explain to young scientists that today they are going to make some ghosts disappear!
- Invite them to touch the ghosts and guess what material was used to make them
- Talk about the texture, the weight, and ask if it reminds them of anything they’ve seen/touched before
- Show them how to use their pipette and tell them to carefully drip some water onto their ghosts
- What happens?!
- You can also use a paintbrush to carefully drip water
- What happens?!
- Eventually they will notice that their ghosts are shrinking and “disappearing” into a pool of water!
- Ask them what happened? How do they think that happened? Do they think the ghosts really disappeared? Where did they go?!
The Science:
Although it may have LOOKED like the ghosts were disappearing, they were actually dissolving! The packing peanuts are made of corn, which dissolves in water. If you put a handful of these packing peanuts into a jar of water and shook it, they would completely dissolve--leaving some cloudy white water behind.
Packing peanuts are traditionally made of styrofoam which takes millions of years (we think!) to biodegrade and become dirt in the earth. These packing peanuts biodegrade almost instantly, making them much more environmentally friendly--and they also make an awesome science experiment!
Bonus Activity: Monster Ice!
Can’t get your hands on biodegradable packing peanuts? No worries! Try this fun experiment instead.
Materials Needed:
- Plastic container (freezer safe)
- Water
- Googly eyes or other Halloween trinkets (spider rings, erasers, etc)
- Small cup with warm salt water
- Pipette or paintbrush
- Tray or cookie/baking sheet
- Red & yellow food coloring (optional)
Instructions - Set-up:
- The night before the activity, or several hours before, put water into your plastic container.
- Add yellow and red food coloring to make orange, if you’d like
- Put in the googly eyes or other Halloween trinkets
- Freeze!
- Place plastic container with ice* on a tray with cup of warm salt water & pipette/paintbrush
- *You might be able to get the ice out of the container, if not--start with the ice still in the container and as young scientists add water & salt, you will easily be able to get the ice out!
Instructions - Activity:
- Invite young scientists into the experiment area
- Ask them what they see!
- Encourage young scientists to use the pipette or paintbrush to drip warm salt water onto the ice and “free” the halloween trinkets
- Ask: What is happening?
- Mention that the water is warm and has salt in it--ask how they think this might help to melt the ice
- Continue adding water until the trinkets are unfrozen!
The Science:
- Salt lowers the freezing point of water. Ice melts faster when salt is added as the salt lowers the freezing point of the ice, this is known as freezing point depression. The more salt you add the lower the freezing point.
- This is why we use salt on roads in the winter to help melt the ice and make them safe!
Spooky Sparks
by Meredith Brustlin, CMNH Educator
Materials Needed:
- Oven safe dish - reusable or disposable aluminum
- Steel wool
- 9V battery
- Jug of water - just in case!
Instructions & Safety:
- Since this activity does produce smoke, you may want to set-up outside. Just be sure if you do this experiment outside that it is not a windy day.
- I did this experiment inside my house several times and there was not enough smoke to set off any smoke detectors! HOWEVER I had coarse grade steel wool so the circuits & smoke were far less significant. If you have FINE grade steel wool, do this experiment outside.
- If you have young scientists--do this experiment as a demonstration (“I’m going to do it, and you get to watch!”)
- If your scientists are older, they can do this experiment but make sure they are closely supervised.
- The steel wool produces sparks & fire
- The 9V battery will eventually get fairly warm to the touch.
Set-up:
- Pull apart your steel wool so that it is in a very thin layer
- Place the steel wool into your oven safe dish or container
- Place the battery and jug of water nearby.
Activity:
- Take time at the beginning of this experiment--whether you are doing it as a demonstration or young scientists are participating--to discuss safety.
- Ask scientists what they see. Invite them to feel the steel wool. What does it feel like? Does it remind them of anything?
- Explain that today you are going to make spooky sparks by creating circuits within the steel wool.
- Use the battery to gently touch down on the steel wool---watch as sparks fly through the steel wool creating a chemical reaction!
- Keep creating circuits! Eventually you will “use up” all the steel wool and the circuits won’t work anymore. Also be conscious of the battery warming up--it’s working hard!
The Science:
We are seeing a chemical reaction take place in this experiment, Anytime something burns, we are seeing a chemical reaction! This type of chemical reaction is called a combustion reaction.
You are seeing the steel wool react with oxygen and in this case it is forming iron oxide.
We were also seeing circuits at work! When both battery terminals touch the steel wool, the electrons from the battery move rapidly through the steel wool and make a complete circuit. This electrical current is heating up the wire (to ~700 degrees!) and this heat causes the iron to react with the oxygen surrounding the little strands of steel wool. This reaction is what causes the sparks (homeschoolscientist.com).
Bonus Activity: Fizzy Pumpkins
Materials Needed:
- Baking Soda
- Water
- Red & yellow food coloring
- Small piece of cardboard
- Tray or cookie sheet/pan
- Pipette or paintbrush
- Small cup of vinegar
Set-up:
- Make your fizzy pumpkin!
- Measure out about ½ a cup of baking soda into a bowl
- Slooooowly add water until the baking soda comes together to form a moldable paste
- Add yellow and red food coloring to make orange and mix together
- Use your hands to shape the baking soda into a ball
- Push in the piece of cardboard in the top to look like the stem of a pumpkin
- **You can either make your pumpkin immediately before doing your experiment, or make it the night/several hours before**
- If you make it right before your pumpkin will be “mushy” but still hold its shape
- If you make it hours before doing the experiment, the baking soda will dry out and become hard as a rock!
To set-up your experiment area, put the pumpkin on a plate or tray and set the cup of vinegar and pipette/paintbrush nearby.
Activity:
- Invite young scientists into the experiment area
- Ask them what they see!
- If you’d like, you can tell them they can carefully touch the pumpkin with one finger and guess how it was made/what material was used to make it.
- Introduce the vinegar and pipette/paintbrush and tell young scientists to carefully drip some vinegar onto their pumpkin
- What happens?!
- Keep playing until the pumpkin turns into pumpkin mush!
The Science:
This is a classic acid and base experiment. When vinegar (an acid) interacts with baking soda (a base) we get a chemical reaction. In this case we’re producing a gas (carbon dioxide) and lots of fun fizzing and bubbles!
Spooky Science: Frankenstein's Hand and Fizzy Pumpkin Art
by Colie Haahr, CMNH Educator
In this Spooky Science Video we make Frankenstein's "hand" come to life with a simple chemical reaction! We also demonstrated an art project using the same materials to make fizzy pumpkin art. Most of the materials for this project can be found at home, and though it's a bit messy, these are both activities that children can try on their own with just little help from a grown-up.
Frankenstein's Hand Experiment
Materials needed:
- Cup
- Disposable glove
- Baking soda
- Lemon juice or vinegar
- Tray or pan
- Scoop or spoon
Set up:
Put all the materials on a tray or another surface that can get wet. This is a good activity to try outside for an easier clean up. The ingredients we are using are actually used for cleaning, which means they should not stain surfaces. A tray is best if you have one. If desired you can pour the lemon juice or vinegar into the cup ahead of time, or children can help with this step.
Experiment:
- Place the cup on the tray, and fill it about ¼ of the way up with lemon juice or vinegar- this experiment works with either one
- Use a spoon to place a few scoops of baking soda into the glove
- Shake the glove so that the baking soda moves down to the fingertips
- Carefully place the glove on top of the cup by stretching the elastic opening of the glove around the mouth of the cup- the fingertips should be hanging down still
- Be sure there’s a tight seal between the glove and cup- if not, hold the glove in place
- Tip the glove upright so that the baking soda drops down from the fingertips into the cup
- Watch the glove! It should start to inflate quickly as the baking soda reacts with the lemon juice inside the cup
Optional Alternative way to do this experiment:
If you do not have a glove handy you can also do this same experiment with a balloon and a plastic bottle. First, draw a face on the balloon, so that when it expands you will have a funny face or monster face on your project. The rest of the steps are the same, except you are placing the baking soda in the balloon, and carefully attaching it to the top of the water bottle. The reaction will be the same as well- the balloon will magically inflate on its own! The Children’s Museum of Indianapolis shared a video of this version of the experiment: Monster Balloon Experiment. Another resource for this experiment comes from Scholastic: Fizzy Balloon Experiment
How does it work?
Lemon juice is acidic and baking soda is a base, and when acids and bases mix together, they fizz up! You created a chemical reaction inside the cup: you mixed together a liquid and a solid, and released a gas: carbon dioxide. When you add the baking soda to the lemon juice the gas starts to move out of the cup, but the glove is blocking it from escaping. The glove inflates from the gas, and will stay inflated while the reaction continues to happen. This experiment is fun because you can “see” the gas that’s created by trapping it inside the glove. Usually, we can’t see the gas that is released from a baking soda and vinegar (or lemon juice) experiment.
Fizzy Pumpkin Art
Materials:
- Tray or placemat to work on
- Orange food coloring or liquid watercolor (mix yellow and red to make orange if needed)
- Lemon juice or vinegar
- Baking soda
- Pipette or spoon
- Pumpkin template- or draw your own with a permanent marker- works best on cardstock. Here are a few free printable pumpkin templates from FirstPallete.com: Medium Sized Pumpkin Template, Large Pumpkin Template
Set Up:
Place the pumpkin template on the tray or work surface. If you would like, use a permanent marker to draw a jack-o-lantern face on the pumpkin. Pour the lemon juice or vinegar into a cup and add the orange food coloring. If you do not have orange, mix red and yellow food coloring together to make it. Keep in mind that the color will become lighter when it mixes with the white baking soda. Spread a light layer of baking soda over the pumpkin image.
Activity Instructions:
- Once everything is set up, use the pipette to add lemon juice to the pumpkin picture
- You should see the baking soda on the pumpkin starting to fizz up! Continue to add lemon juice or vinegar until the fizzing stops
- Let your project dry, and it will become a nice art project. If there is too much liquid on the picture, either use the pipette to remove some, or gently shake it off
- Once dry, you can gently brush off any excess baking soda that is left.
- This activity uses the same ingredients as the experiment, and the chemical reaction is the same. The only difference is that we added some color to the lemon juice or vinegar, so just keep in mind that this will need more clean up, and that food coloring may stain surfaces or clothing.
Walking Rainbow Experiment

By Meredith Brustlin, CMNH Educator
We share a lot of different science experiment projects at CMNH. Many of these experiments have instant results - things like vinegar and baking soda bubbling, popcorn dancing, and invisible ink appearing. What I really like about this “walking rainbow” experiment is that it takes some time to see the results (as in, it takes hours to fully see the results!) Although it may not be as exciting in the moment, it is a perfect experiment to practice making predictions and thinking like a scientist. It really gives young scientists time to think and hypothesize and even change their guesses throughout the day.
To make your own rainbow you will need:
- 7 clear cups--these can be glass or plastic, just make sure they are perfectly clear (not colorful)
- Water
- 6 paper towel pieces (this could be three large sheets cut in half, or 6 of the little half sheets)
- Primary colors of food coloring or liquid watercolor
Set-up:
- Set out the 7 cups in a line
- Fill cups 1, 3, 5, & 7 about halfway with water
- Leave cups 2, 4, & 6 empty
- Fold your six paper towel pieces in half lengthwise and then in half again. Take that long skinny strip and fold it in half so that it can stand up in the cups (pictured above)
- Invite young scientists to help you add some food coloring to the cups.
- Ask them--what is the first color of the rainbow? Red! Add about 6 drops of red food coloring to the first cup.
- What is the second color? Orange! That cup is empty though, so we will skip that one.
- Add yellow to the 3rd cup
- Skip the 4th cup (green)
- Add blue to the 5th cup
- Skip the 6th cup (purple)
- Add red to the 7th cup and ask your scientists why they think you may have done that…!
- Next, carefully place your paper towel pieces in between each cup so that they are resting in the colorful water.
That’s it for set up! Now it’s time to think like a scientist!
Ask your young scientists…
- What do you think will happen with the cups?
- Why do you think we left some cups empty?
- What do you know about primary colors?
- How do you think what you know about primary colors (that they make secondary colors) will come into play in this experiment?
- What do you notice is already happening with the paper towel pieces and the water?
Feel free to have your scientists write or draw what they are seeing!
This experiment will take awhile to completely finish and make the rainbow design. It’s a good idea to either do this experiment at night before bedtime, make some predictions, and then wake up and see the walking water rainbow. You could also do this experiment first thing in the morning and then watch it change all day long!
Have fun making a walking rainbow and thinking like scientists!
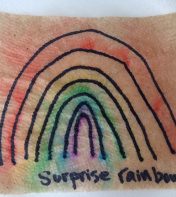
Science Magic: Secret Rainbow
By Colie Haahr, CMNH Educator
This project is fun and easy, and only requires a little bit of prep ahead of time. Kids will enjoy magically making a rainbow appear by adding water to a paper towel. This activity is fun on its own, or it can be set up as an experiment that they can try a few times and record the results.
Materials Needed:
Paper towels
Washable markers
Black permanent marker
Cup for water
Tray or plates
Pipette, eye dropper, or small spray bottle
Optional: Different brands of markers, highlighter, permanent markers, pencil, pen
Directions:
- Prep ahead: This project requires some prep ahead if you want to create a truly “secret” rainbow! You can create more sheets for kids to try as you go if they enjoy the activity! -Use a water based marker to create a rainbow on a paper towel. Remember ROYGBIV!
-Thin lines work well for this- do not make them too thick because you need to cover them with permanent marker
-Use a black permanent marker to carefully draw over each line, hiding the colorful lines under the dark lines.
-Optional: create shapes that are different colors, and use the same process to cover them with permanent markers.
-Optional: Set up the color mixing part of the activity the same way coloring two circles next to one another in primary colors (red and yellow, red and blue, and yellow and blue)
- Place one of the prepared paper towels onto a plate or tray that is okay to use for an art project (it may get marker ink on it!), with a cup of water and pipette or spray bottle
- Make a prediction- ask: what do you think will happen when we add water?
- Ask kids to slowly add some water to the picture
- The rainbow will “magically” appear when the water based marker dye spreads, and the permanent ink stays in place
- Try the same method with color mixing, or the colors hidden behind shapes
- Let the artwork dry completely, and some of the dye will stay on the paper towel once it dries. Too much water can cause the color to wash out completely
- Alternatives: This also works with a tray of water, and kids can carefully place the paper towel in the water and watch what happens.
-Make an experiment! To make this a true experiment, try using different types of writing utensils, like markers, pens, pencils, highlighters, etc., and testing each one to see if water changes anything. Compare different brands of markers to see which ones work the best. Make predictions and write down the results!
Science Information:
For this activity there were two types of markers used, and they were made of different types of dyes or inks. One was water soluble, and one was not. If the experiment went as planned, the water based markers should have spread color out on the paper towel. These markers have dye that is water soluble, which means that it is able to dissolve in water.
The permanent marker has a different kind of ink or dye, and it is not water soluble, which means that water will not wash it away. The permanent marker is alcohol based, so it will dissolve in alcohol. If rubbing alcohol were added to the paper towel, it may have changed the permanent marker, but because only water was added, the permanent marker stayed the same.
The paper towel also played an important part in making this activity work. This activity did not work well on other types of paper, like card stock and copy paper. Paper towels are absorbent, and designed to absorb or suck up liquids. If the paper were waxed paper or something similar, the water would bead up into droplets because water molecules like to stick together. Water molecules still like to stick together on paper towels, but there are tiny air pockets in paper towels, and a soft cellulose material that allows the water molecules to move around the paper towel together.
References:
https://indianapublicmedia.org/amomentofscience/how-do-paper-towels-absorb-water.php
https://www.thebestideasforkids.com/surprise-rainbow-activities/
Oil & Water Mixing Sensory Bottles
by Meredith Brustlin, CMNH Educator
There are so many fun experiments that you can try using oil and water. If you’re at the grocery store and thinking about picking up one or two items for your young scientist’s “at home chemistry station” I definitely recommend a big jug of vegetable oil. It’s inexpensive, easy to find, and can be used for tons of science!
This experiment does some simple MESS FREE oil and water mixing. I especially like this experiment because the experiment itself is quick and can be done over and over again. Some people also use these oil & water mixing bottles as sensory bottles - moving the oil back and forth and watching the gentle waves it makes can be very relaxing. Who doesn’t need a bit of extra relaxation these days?
Here’s how to make your own:
Materials needed:
- Small jar or container with a lid that can be securely screwed on (plastic or glass--although with very young scientists you may want to go the plastic route and duct tape that lid on there, too!)
- Vegetable oil
- Food coloring
Directions:
- Fill your container halfway with water
- Pour vegetable oil in to fill up the rest of the container
- Watch and see what happens!
- Add a drop of red or blue food coloring
- Watch closely again!
- Tightly secure the lid of your container
- Optional: add some duct tape to really seal it in place
- Watch as the oil and water in your bottles become completely separate and the drop of food coloring makes its way down to the water portion of the bottle - it will take a few seconds.
- Gently move the bottle around and watch the oil and water - they stay separate when moved gently
- SHAKE your bottle! Really shake it and watch as the water and oil temporarily mix
- What else happens?!
- Watch closely again as the oil and water slowly separate
The Science:
There are several different science “happenings” going on during this experiment.
Oil & Water:
- Your scientist’s will notice that the oil and water do not mix! The oil sits on top of the water and it always will. This is because water and oil are immiscible. Basically what this means is that water molecules only want to hang out with other water molecules and the same with oil molecules. The oil sits on top because it is less dense or less heavy than the water so it happily floats on top of it.
Primary/Secondary Colors:
- Vegetable oil is used for this experiment because it is yellow in color. If we used another kind of oil, like baby oil, you would have to purchase oil based food coloring which isn’t all that easy to find. The yellow vegetable oil automatically gives us one of our primary colors. Blue or red food coloring is added so that when you mix, you get a secondary color!
- There are lots of great books you can read/find videos of online to explore primary/secondary colors, check out:
- Mouse Paint by Ellen Stoll Walsh
- Mix It Up by Herve Tullet
- Monsters Love Colors by Mike Austin
- There are lots of great books you can read/find videos of online to explore primary/secondary colors, check out:
Science Magic: Glitter & Soap
by Colie Haahr, CMNH Educator
Try out this easy experiment with materials you already have at home! This experiment is about surface tension, and you can make glitter magically “dance” in a bowl of water! The reaction is quick, but kids love trying it more than once. This could be a good experiment to try before transitioning to water play, which always seems to be a hit!
Materials Needed:
Shallow bowls or plates
Water
Glitter, Pepper, Cinnamon, or All Spice
Toothpicks or Q-tips
Dish Soap
Toothpaste
Cooking Oil
Directions:
- Set up: Pour water into bowls, and place a very small amount of all of the other liquids into lids or small bowls. A pitcher of water is helpful to reset the experiment. The experiment works best with dish soap, but using a few other substances makes it more of a true experiment, where some will work and some will not.
- Optional: have a pencil and paper handy to record observations and hypotheses
- Pour about a teaspoon of glitter into one bowl of water, and a teaspoon of whatever spices you would like to use into another. You want the glitter and spices to cover the surface of the water
- Make an observation: what happened when we poured the glitter/spices into the bowl?
- The glitter or spices stay on the surface of the water because they are hydrophobic, and they do not dissolve in water like salt or sugar would.
- Carefully dip the end of a clean toothpick or Q-tip into the liquid dish soap, and poke it right into the center of the bowl
- Make an observation: What happened to the glitter/spices? The glitter should move quickly to the edges of the bowl when the soap touches it.
- Repeat the process with the toothpaste, cooking oil, hand soap or anything else you decide to try
- Optional: write down what happens each time you try the experiment
Science Information:
Water molecules like to stick together, so when you pour a drop of water onto something non-porous, like waxed paper, the water beads up. Kids usually can picture this happening on a windshield when it’s rainy the rain drops stick together and roll down the windshield.
When you pour water into a bowl or plate, this creates surface tension.
During the experiment, you observed that the glitter and spices in the water bowls stayed right on top. Even though water molecules like to stick together, they do not always stick to other things, like the glitter and spices. The surface tension of the water allows these small particles to float on top! They do not dissolve, and usually, they do not get saturated and sink.
When you added different substances to the water, some caused the glitter and spices to move away to the sides of the bowl. Now experiments can be tricky, and they do not always work perfectly, but the oil should have made no changes to the water bowl, and the soap and toothpaste should have caused the particles to move. The substances that made the glitter and spices had something in common: they all clean things!
Dish soap should have worked the best, and this is partially because dish soap has molecules (teeny tiny parts) that are BOTH hydrophobic and hydrophilic. Wait, that would mean the soap molecules repel water molecules, and attract or bond to them! This is true, soap is a good cleaner because it can pull things like oil out of water because of the hydrophilic properties, like when we wash dishes, dish soap helps to get rid of grease and oil that water alone can’t remove.
When the soap touched the water bowl, it broke the surface tension of the water, and that’s why we could see the glitter and spices move. Soaps and cleaners are designed to break down the surface tension of water. This helps make them good cleaning tools. When you added the dish soap or toothpaste to the water it broke up the surface tension. The water molecules, however, want to stick together and maintain that tension, so they move away from the soap, carrying the glitter and spices with them! We can see the reaction because there are particles floating on the top of the water. The water would still move when soap is added, but because it is clear, we can’t see it. The glitter and spices help us see what’s happening in the water bowl!
Science MAGIC: Invisible Ink!
By Meredith Brustlin, CMNH Educator
I was THRILLED to find this invisible ink recipe. Many invisible ink recipes involve holding dried lemon juice messages over a candle and almost setting pieces of paper on fire. Luckily, this recipe doesn’t require any heat and is totally safe---besides the slightly strong scent of rubbing alcohol. However, with it warming up outside--this would be a great outside project!
Here’s how to make your own heat-free invisible ink:
Materials needed:
- 2 medium-size glass containers (I used Pyrex liquid measuring cups)
- Q-tips
- Paintbrushes
- Paper (white paper works best, you could also use cardstock!)
- Table covering
- Cookie sheet/craft tray
- 1 tsp powdered tumeric
- ½ cup rubbing alcohol
- 1 Tbsp baking soda
- ½ water
Directions (prep):
- Set up your experiment area, whether inside or outside, by protecting the area with some kind of covering. This could be a plastic tablecloth, trashbag, or some other non-absorbent material.
- Place a cookie tray in the experiment area
- Gather all other materials and have them on hand.
Directions (activity):
- Tell little ones that today you will be experimenting with invisible ink!
- Invite them to help you mix 1 Tbsp of baking soda into ½ water in one of your glass containers
- Mix until it is mostly dissolved and keep mixing throughout your experimenting--it doesn’t dissolve all that quickly.
- Have little ones draw on their paper with the baking soda solution using q-tips
- It will be hard to see what they are drawing! They can try writing words or just doing abstract doodles. Anything will be fun to find using the invisible ink decoder!
- Put aside the drawings to dry
- While they are drying, make your “decoding” solution
- Mix 1 tsp turmeric powder into ½ cup of rubbing alcohol
- The turmeric solution will stain hands and surfaces--so be careful while mixing and using this solution. At least it won’t set your house on fire, right?!
- Once your papers are completely dry, place them on the cookie sheet and paint over them using the tumeric decoding solution. What happens?!?!
- Watch your paper change color more as they completely dry.
The Science:
(For younger scientists):
- Explain that the baking soda “ink” is changing color because it reacts or changes when it meets the turmeric solution. There is an ingredient in the turmeric that changes the baking soda to that very deep purple color when they meet!
(For older scientists):
- Turmeric is a ph indicator. This means that it will change the color of different substances when it interacts with them to show us what their ph is.
- Ph tells us the acidity or basicity of items.
- Basically, substances go through a chemical reaction when they “meet” a ph indicator and that causes them to change color.
- Think about a traditional baking soda and vinegar experiment - they combine and erupt! That is because baking soda is a base and vinegar is an acid. If we tested the ph of vinegar it would be a very different color than the ph of baking soda.
- When you paint over the baking soda papers with turmeric, we are seeing that deep purple appear because that is the color that baking soda changes when it interacts with a ph indicator.